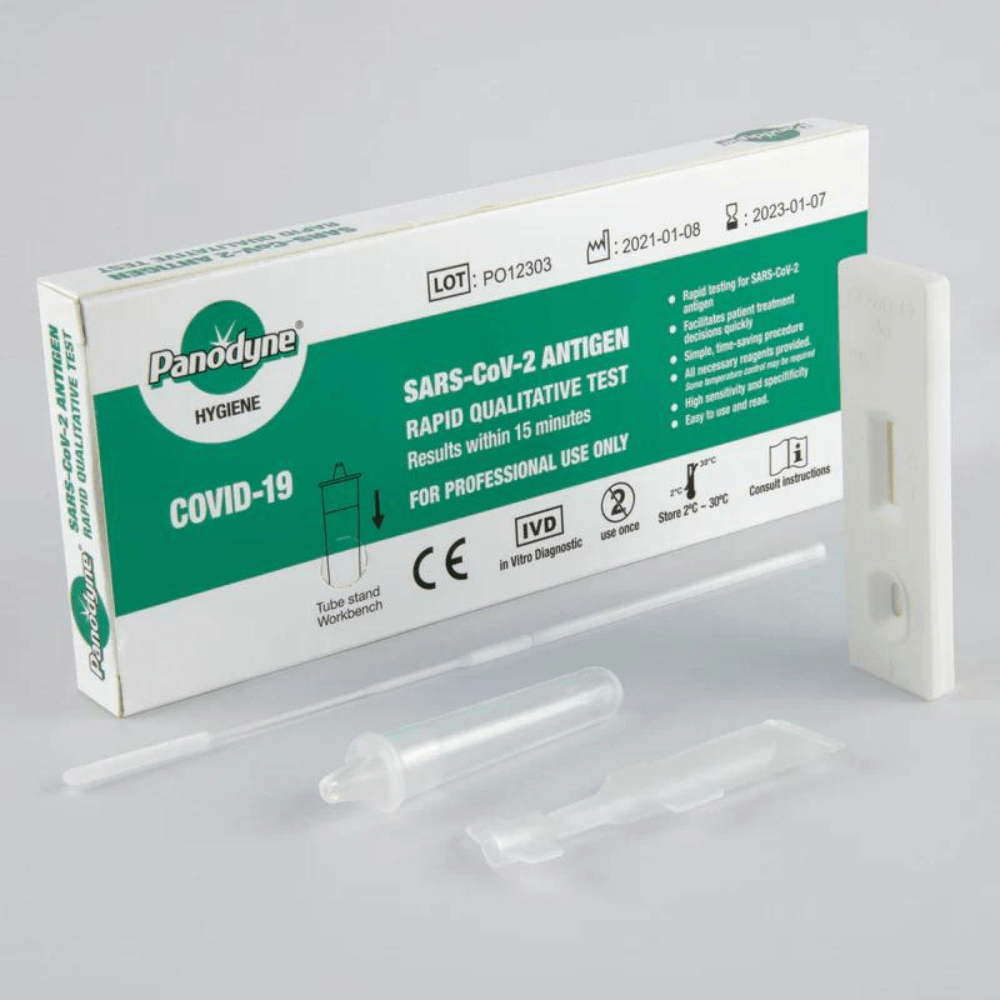
Panodyne Sars Cov 2 Antigen Rapid Test Box of 24
The novel corona viruses belong to the β genus. SARSCoV-2 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel corona virus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhoea are found in a few cases.
Antigen is generally detectable in upper respiratory specimens during the acute phase of infection. Rapid diagnosis of SARS-CoV-2 infection will help healthcare professionals to treat patients and control the disease more efficiently and effectively.
Benefits
- Rapid testing for SARS-CoV-2 antigen within 15 minutes
- Facilitates patient treatment decisions quickly
- Simple, time-saving procedure
- All necessary reagents provided and no equipment needed
- High sensitivity and specificity
KIT CONTENTS
- Test cassette
- Sterile swab
- Extraction tube
- Antigen extraction buffer




Reviews
There are no reviews yet.